 To keep a competitive edge, the push is on from lab managers and principal investigators to purchase equipment of their own. CBS Labs Genomics Department in Miami offers practitioners in search of the latest modern sequencing technology access to a full line of sequencing technologies coupled with targeted oncology panels providing accurate data, analysis, reliable support, and scalability.
To keep a competitive edge, the push is on from lab managers and principal investigators to purchase equipment of their own. CBS Labs Genomics Department in Miami offers practitioners in search of the latest modern sequencing technology access to a full line of sequencing technologies coupled with targeted oncology panels providing accurate data, analysis, reliable support, and scalability.
Understanding genetic changes in cancer involves investigating all stages of tumor progression pathways. With CBS Labs Genomics Department, NGS panels track body cell progressions, from abnormal tissue growth changes to tumors, response and recurrence.
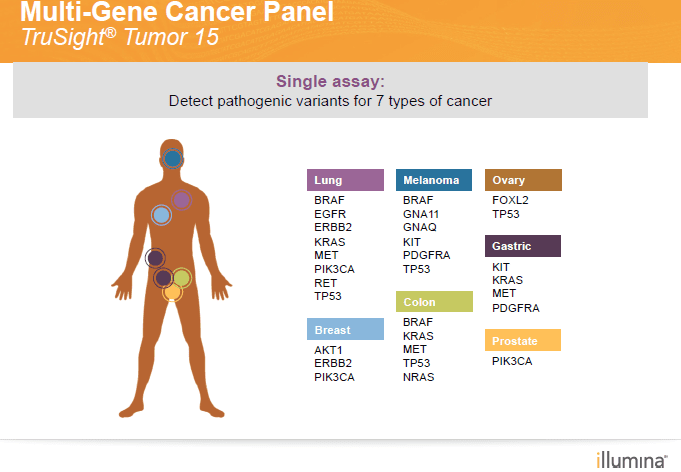
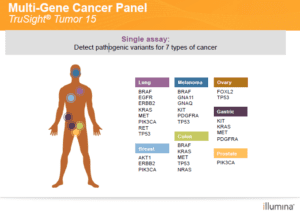
The diagnostic tool at the forefront is the TruSight® Tumor 15 assay, a multi-gene cancer panel delivering focused gene content that replaces repetitive tumor profiling. From sample to report, this cutting edge, comprehensive method provides highly sensitive detection of important variations of cells in solid tumors.
CBS Labs’ TruSight Tumor® 15 multi-gene panel can detect pathogenic variants in seven types of cancer: Lung, Melanoma, Ovary, Gastric, Breast, Colon, and Prostate. The process is four-step, from sample collection and library workflow prep to sequencing and analysis.
The TruSight Tumor® 15 assay derives its name from its ability to use NGS to assess 15 of the most commonly mutated genes in solid tumors.
In addition to its extraordinary diagnostic benefits, CBS Labs NGS systems are cost-efficient. By using NGS to examine multiple cancer-associated alterations side by side lowers costs and delivers faster turnaround times compared to repetitive single-gene analysis due to the accelerated tumor profiling of NGS.
Among the newest next generation sequencing tests from CBS Labs are BRCA1 and BRCA2, a genetic test for harmful mutations faced by women at risk for developing breast or ovarian cancer. While recent studies have determined about 12 percent of the general population will develop breast cancer, those with the harmful mutation pose a risk upwards of 65 percent. Candidates for testing include those with existing breast cancer and women with a close relative who have a harmful BRCA mutation.
CBS Labs Genomics Department uses bioinformatics software that accesses the most up-to-date and reliable genomics databases so not only do practitioners receive expert bioinformatics review of the sequencing data, the CBS Labs report also lists relevant clinical trials that may be available to patients.
Also new is CBS Lab’s immunohistochemistry test- a staining process preformed on fresh or frozen cancer tissue removed during biopsy-known as PD-L1 Immunoassay, with applications for bladder cancer, lung cancer and melanoma.
CBS Labs was founded in 2001 in response to South Florida’s need for high quality, local laboratory services.